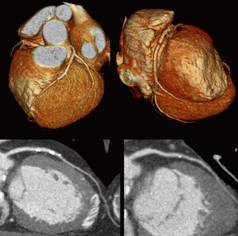
Cardiac CT showing calcified plaques.
Calcium plays a central role in the electrical stimulation of cardiac cells and in the mechanical contraction of smooth muscle cells in the wails of arteries.
Calcium is essential to health yet it holds a hidden danger that brings us to our graves much quicker then we would like. Calcium is the most promoted nutrient by proponents of conventional, nutritional, and alternative medicine. This is a great and tragic mistake. They should have been promoting magnesium. Magnesium deficiency leads to an increase in myocardial levels of both sodium and calcium. This is a problem because Coronary Artery calcium is a predictor of near-term coronary heart disease events. In the face of growing magnesium deficiencies calcium becomes increasingly more toxic to human physiology.
Dr. Dean makes this clear when she says in her book The Magnesium Miracle, “To understand how you can create a calcium/magnesium imbalance in your own body, try this experiment in your kitchen. Crush a calcium pill and see how much dissolves in 1 oz of water. Then crush a magnesium pill and slowly stir it into the calcium water. When you introduce the magnesium, the remaining calcium dissolves; it becomes more water-soluble. The same thing happens in your bloodstream, heart, brain, kidneys, and all the tissues in your body. If you don’t have enough magnesium to help keep calcium dissolved, you may end up with calcium-excess muscle spasms, fibromyalgia, hardening of the arteries, and even dental cavities. Another scenario plays out in the kidneys. If there is too much calcium in the kidneys and not enough magnesium to dissolve it, you can get kidney stones.”
Magnesium and calcium work together to control muscle action though calcium becomes a problem when there is not enough magnesium to control calcium’s actions. Calcium becomes a slow acting poison (often decades of build up) to tissues all over the body when in excess relative to magnesium in deficiency. Trace mineral symptoms of excess or deficiency depend on their ratios to other elements. In the event of calcification, it is not particularly a high calcium level that results in the formation of a stone or spur, but calcium being high in ratio to magnesium.
Magnesium increases the solubility of calcium in the urine. Supplementing magnesium to the diet has demonstrated significant effect in preventing recurrences of kidney stones.
If calcium is not taken with magnesium or it if it is not highly absorbable, it will cause much more harm than good. Unabsorbed calcium can lodge anywhere in our body. For instances, if it lodges in your bones and joints, it mimics arthritis; if it lodges in you heart, it mimics arterial lesions. Calcification or calcium poisoning can manifest as heart disease, cancer, wrinkled skin, kidney stones, osteoporosis, dental problems, bone spurs, cataracts and many other health problems. Calcium deposits in the joints are called arthritis; in the blood vessels it is hardening of the arteries; in the heart it is heart disease, and in the brain it is senility.
It is magnesium that actually controls bone density not calcium. Magnesium drives the calcium into the bones where low levels encourage its loss.
Exceedingly few healthcare practitioners in the world have learned much about magnesium medicine so they do not know to lay off the calcium and start intensive magnesium treatments. After decades of dairy industry marketing pushing calcium we have a situation that is literally killing millions of people. Anyone who wants to live longer should pay attention to the magnesium story and should immediately begin a strong and prolonged treatment with magnesium in its chloride form. Magnesium chloride is the most versatile, absorbable and effective form of magnesium and can be used orally, transdermally and via IV drip. It can even be nebulized directly into the lungs and in much diluted form dropped into the eyes when its purest forms are used.
While calcium affects muscle contractions, magnesium balances that effect and relaxes muscles. Calcium tightens the muscles; magnesium relaxes the muscles. With insufficient magnesium the muscles stay tense and through the years may cause a cramp in the muscle. This could happen when you have too much calcium or too little magnesium. Too much calcium causes the heart to go into a spasm and it can’t relax. This is a heart attack. Get some magnesium into the body and the heart will slowly start returning to normal unless major damage has already been done. Add iodine and selenium and we have the makings of an ideal formula to support recovery and possibly even minor tissue regeneration.
As we will see in another chapter medical scientists are already creating heart patches made from sea weed and are seeing both blood and heart tissues growing and regenerating into the patches. Seaweed just happens to be high in magnesium, iodine and selenium. A great part of this book will be devoted to mercury poisoning and the tendency of it to be concentrated in cardiac tissues. Selenium is the antidote to mercury and iodine reveals one more of her secrets when it comes to cardiac care.

Characterization of liver calcification. Von Kossa (top panels), alizarin red S
(middle panels) and Goldner–Masson trichrome (bottom panels) staining of
calcified, precalcified and noncalcified liver tissue sections. Top panels, black
staining indicates the presence of phosphate precipitate. Middle panels, dark grey
staining indicates the presence of Ca2+ precipitate. Bottom panels, light grey
staining indicates the presence of collagen. Magnification 20.
There are no pharmaceutical drugs on the market to reduce calcium deposits but magnesium chloride and sodium thiosulfate are useful in preventing and treating unwanted calcification. Together they offer the best way of combating the calcium time bomb going silently and slowly off in uncounted millions of people. The best way to track calcium toxicity is actually through looking at the level of deficiency in magnesium for magnesium controls and counteracts calcium. The average American consumes only 40 percent of the recommended daily allowance of magnesium. This has serious consequences, including death, in many people, according to magnesium expert Dr. Mildred Seelig. Eighty to 90 percent of the U.S. population is magnesium deficient.
Calcification consists of calcium and phosphorous and is a normal process for building healthy bones and teeth. But it also plays a central role in disease conditions such as strokes and heart attacks.
Dr. H. Ray Evers writes, “The power plant of human cell is called the “mitochondrion.” The mitochondrion is what generates energy for the cell to use. What everyone refers to as “energy” is derived from the oxidative reduction of the cellular respiration. This is done through the mitochondria. But the problem arises when the cell is low in magnesium, relative to calcium. Adenosine triphosphate, the “energy currency” of the cell, is magnesium dependent. This means it is obvious that the calcium pump at the cell membrane is also magnesium dependent. Without enough “biologically available” magnesium, the cellular calcium pump slows down. Thus a vicious cycle is established. The low levels of available magnesium inhibit the generation of energy, and the low levels of energy inhibit the calcium pump. The end result? The mitochondrion, the powerhouse of the cell and the entire body, becomes calcified. This is the beginning of aging. It all starts in the cell. First the cells age. This leads to organ aging. And after the organs age, individual aging occurs. Since calcium is readily accumulated by mitochondria, this ion is potentially capable of antagonizing the activating influence of magnesium on many intra-mitochondrial enzyme reactions. This means that every function of your body can be inhibited when the mitochondria calcify. It’s like going through life with the emergency brakes on. Calcium is the brake. Magnesium is the accelerator. To be in optimal health, there must be a balance between the two.”
The higher the protein you consumer the more magnesium is needed. When large amounts of calcium are consumed, you need more magnesium. A diet which is high in calcium increases the body’s need for magnesium.– Dr. H. Ray Evers
The higher the calcium level and the lower the magnesium level in the extra-cellular fluid, the harder it is for cells to pump the calcium out. Mitochondria produce the energy our cells and organs need. This is vitally important for the heart because heart muscle cells have a never ending need for energy. Mitochondria are also important for proper neurotransmission and are highly concentrated in cells of the brain and central nervous system. A healthy cell has high magnesium and low calcium levels.

Calcifying Nanoparticles (CNPs) form slow-growing calcified colonies
in arteries and organs, much in the same way as coral reefs form.
We may say that our biochemical age is determined by the ratio of magnesium to calcium within our cells. As we age, calcium deposits tend to accumulate in our soft tissues. Doctors call it “Extra-skeletal calcification.” This means that the calcium that is supposed to be deposited in your bones is being lodged in our soft tissues.
Up to 30% of the energy of cells is used to pump calcium out of the cells.
Deficiency in magnesium, aside from having a negative impact on the energy production pathway required by mitochondria to generate ATP, also reduces the threshold antioxidant capacity of the cardiovascular system and its resistance to free-radical damage. Magnesium acts as an antioxidant against free radical damage of the mitochondria.
Magnesium has been called nature’s “calcium channel blocker” because of its ability to prevent coronary artery spasm, arrhythmias, and to reduce blood pressure.
“Calcium enters the cells of the heart by way of calcium channels that are jealously guarded by magnesium. Magnesium, at a concentration 10,000 times greater than that of calcium in the cells, allows only a certain amount of calcium to enter to create necessary electrical transmissions, and then immediately helps to eject the calcium once the work is done. Why? If calcium accumulates in the cell, it causes hyperexcitibility and calci?cation and disrupts cell function leading to angina, high blood pressure, arrhythmia, asthma, headaches and even heart attacks. Magnesium is nature’s calcium channel blocker,” says Dr. Carolyn Dean, author of The Magnesium Miracle.
Dr. Garry Gordon wrote, “If you have compromised cell membranes or low ATP production for any reason, then the cell has trouble maintaining the normal gradient. This is because the usual gradient is 10,000 times more calcium outside of cells than inside; when this is compromised you will have increased intracellular calcium, which seems to always happen at the time of death. Whenever intracellular calcium is elevated, you have a relative deficiency of magnesium, so whenever anyone is seriously ill, acute or chronic, part of your plan must be to restore magnesium, which is poorly absorbed through oral means.”
The optimal blood serum value for vitamin D
is 45-52 ng/ml (115-128 nmol/l).
The adverse effects of excessive calcium intake may include high blood calcium levels, kidney stone formation and kidney complications.[1] Elevated calcium levels are also associated with arthritic/joint and vascular degeneration, calcification of soft tissue, hypertension and stroke, and increase in VLDL triglycerides, gastrointestinal disturbances, mood and depressive disorders, chronic fatigue, and general mineral imbalances including magnesium, zinc, iron and phosphorus. High calcium levels interfere with Vitamin D and subsequently inhibit the vitamin’s cancer protective effect unless extra amounts of Vitamin D are supplemented.[2]
Vitamin D works by lowering insulin resistance, which is one of the major factors in heart disease. It is also used by the thyroid gland, which secretes a hormone that regulates the body’s levels of calcium, which in turns helps regulate blood pressure.
Cardiovascular calcification lesions can lead to the development of myocardial ischaemia, myocardial infarction, impaired myocardial function, congestive heart failure, cardiac valve insufficiency, and cardiac arrhythmias. There is a strong association between increased cardiac calcification and risk of death. Administration of vitamin D to treat secondary hyper-parathyroidism increases intestinal absorption of calcium and phosphorus. It raises serum calcium and phosphorus levels. Soft-tissue and vascular calcification are associated with a history of vitamin D therapy.[3]
Changes in serum calcium do provide important information about various hormonal or organic disturbances, including excessive Vitamin D status.
Magnesium and calcium share a common route of absorption in the intestinal tract and appear to have a mutually suppressive effect on each other. If calcium intake (or dairy intake) is unusually high, calcium will be absorbed in preference to magnesium. Also, excessive doses of vitamin D and calcium supplements can cause renal magnesium loss. Sunlight is the only safe way to get vitamin D since the body regulates how much is made. Take it by pill form and calcium homeostasis is overridden. The entire idea of toxic sunscreens and avoiding the sun’s life giving effects (natural vitamin D formation) is just one more mistake modern medicine is making.
Researchers from Winthrop University Hospital in Mineola, New York, found that giving supplements of vitamin D to a group of volunteers reduced episodes of infection with colds and flu by 70 per cent over three years.
The dangers of sun exposure have been greatly exaggerated by the same types of people who over exaggerate and lie about many things in medicine. Sun exposure is not the major reason people develop skin cancer. Researchers point out that increasing level of vitamin D3 could prevent many diseases that claim hundreds of thousands if not millions of people world each year. Vitamin D, the sunshine vitamin, is different from other vitamins in that it influences your entire body — receptors that respond to the vitamin have been found in almost every type of human cell, from the brain to our bones.
Magnesium is essential for the normal function of the parathyroid gland and for vitamin D metabolism.

Coronary artery calcification is common, is severe and is significantly associated with ischemic cardiovascular disease in adult end-stage renal disease patients.[4] The amount of calcium in the coronary arteries reliably predicts heart attack risk and is measured by what is called ones calcium score. UCLA cardiologist, Dr. Matt Budoff, a long-time champion of the Coronary Calcium Scan, and author of the AHA paper says, “The total amount of coronary calcium (Agatston score) predicts coronary disease events beyond standard risk factors.” The Coronary Calcium Score is a precise quantitative tool for measuring and tracking heart disease risk. It is more valuable and accurate than other traditional markers (such as total cholesterol which is practically worthless as a heart disease risk marker).
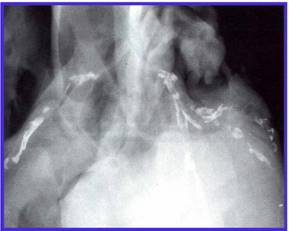
This image demonstrates coronary artery calcification.
According to the University of Florida Shands Cancer Center a high level of calcium in the blood, called hypercalcemia,[5] may become a medical emergency. This disorder is most commonly caused by cancer or parathyroid disease but underneath the primary etiology is a magnesium deficiency. Hypercalcemia is commonly attributed to cancer treatment. Severe hypercalcemia is a medical emergency that can be avoided if magnesium levels are brought up to normal.
Magnesium is the mineral of rejuvenation and prevents the calcification of our organs and tissues that is characteristic of the old-age related degeneration of our body.
Magnesium inadequacy interferes with cellular metabolism and accelerates the aging of most human tissues. Most human cells can only replicate a limited number of times in cultures before they lose the ability to divide, a phenomenon known as replicative senescence. Recent studies have shown that culture in low magnesium accelerates the senescence of human endothelial cells and fibroblasts.[6] Dr. James Howenstein says “Calcification in cellular tissues is a sign of tissue damage, cellular aging and impending cell death. When cells are unable to regulate calcium and keep the calcium content of cells down cellular function degenerates. Calcified arteries, calcium in soft tissues and high levels of calcium within cells are all signs of aging. At age 80 the average calcium content in the aorta is 140 times greater than the levels of aortic calcification noted at age 40.”

Age 20-30 yearsAge 50-70 years
In youth, at left, there is minimal plaque formation. However, at right with passage of time the plaque grows larger. About 20% of this plaque volume contains calcium which is measurable on CAT scan, providing a marker for the total plaque burden. Calcification of atherosclerotic lesions is due to a process of active deposition of calcium in the atherosclerotic plaque that utilizes metabolic pathways similar to those found in normal human bone. Calcium accumulates steadily in plaque and its presence is verifiable via microscopic examination from the very early stages of disease formation. Having a build-up of calcium plaque in the arteries means increased risk of heart attacks and death from heart disease according to findings from the Multi-Ethnic Study of Atherosclerosis (MESA) funded by the National Heart, Lung, and Blood Institute. Researchers at the University of Virginia Health System suggest that composition of plaque deposits in the carotid arteries indicate a patient’s risk of having a stroke.
The ratio of calcium to magnesium in milk is 9 or 10 to 1. Calcium is the physiological partner of magnesium and should be present in a 2:1 or even 1:1 ratio.
American women have been consuming an average of two pounds of milk per day for their entire lives, yet thirty million American women have osteoporosis. Drinking milk does not prevent bone loss. Bone loss is accelerated by ingesting too much protein, and milk has been called “liquid meat.” In order to absorb calcium, the body needs comparable amounts of magnesium. Countries with the highest rates of osteoporosis, such as the United States, England, and Sweden, consume the most milk. China and Japan, where people eat much less protein and dairy food, have low rates of osteoporosis.[7] Dietary protein increases production of acid in the blood which can be neutralized by calcium mobilized from the skeleton.[8] About 50,000 Americans die each year of problems related in some way to osteoporosis.[9]

Aortic valve replacements are done when too much calcification of the heart valve leaflets takes place. According to The Cleveland Clinic, fibro-calcific degeneration most commonly affects the aortic valve. According to reports, calcified heart valves typically occurs in adults over the age of 65. When valve leaflets are calcified, the valve leaflets become fibrotic (thickened) and calcified (hardened), producing a narrowed valve opening. Risk factors for this type of valve disease include increased age, low body weight and high blood pressure.

This photograph shows the aortic valve with a short segment of the aorta around it. The valve clearly has only two cusps (bicuspid aortic valve), and is narrowed and densely calcified. If you placed your fingertip through the opening, the valve would feel hard and gritty.
William R. Quesnell, author of ‘Minerals: The Essential Link to Health, said, “Most people have come to believe nutrition is divisible, and that a single substance will maintain vibrant health. The touting of calcium for the degenerative disease osteoporosis provides an excellent example. Every day the media, acting as proxy for the milk lobby, sells calcium as a magic bullet. Has it worked? Definitely for sales of milk; but for American health it has been a disaster. When you load up your system with excess calcium, you shut down magnesium’s ability to activate thyrocalcitonin, a hormone that under normal circumstances would send calcium to your bones.”
The most common cause of death in dialysis patients is cardiovascular disease. This is due in part to the presence of excess vascular calcification, particularly in the form of extensive coronary artery calcification, which can be observed even in very young dialysis patients.[10] The presence of coronary artery calcification in the dialysis population appears to correlate in part with the ingested quantity of calcium-containing oral phosphate binders.[11]
The associations among valvular calcification,inflammation, carotid atherosclerosis, and arterial calcification suggest that valvular calcification is a marker of atherosclerosis and arterial calcification in patients with end stage renal disease.[12]
Dietary surveys clearly show that magnesium, not calcium, intakes have been falling over the last fifty years. This is a problem because it is magnesium that controls the fate of calcium in the body. If magnesium is insufficient calcium will be deposited in the soft tissues (kidneys, arteries, joints, brain, etc.).
Countries with the highest calcium to magnesium ratios (high calcium and low magnesium levels) in soil and water have the highest incidence of cardiovascular disease. At the top of the list is Australia. Adequate levels of magnesium are essential for the heart muscle. Some researchers predict that the American ratio of calcium to magnesium is actually approaching 6-to-1, yet, the recommended dietary ratio of calcium to magnesium in the United States is 2-to-1. The process of absorption for magnesium is similar to that of calcium but some people absorb or retain much more magnesium than calcium (or more calcium than magnesium). The commonly suggested supplemental intake ratio of 2:1 for calcium and magnesium is arbitrary for the value can change significantly under various individual circumstances.
Current research on the Paleolithic or caveman diet shows that the ratio of calcium to magnesium in the diet that our bodies evolved to eat is 1-to-1.[13] Balancing this information is the fact that mothers breast milk is ten parts calcium to only one part magnesium so it seems that at least early in life we need less magnesium and more calcium to build strong bones Though high doses of calcium carbonate taken alone over a long period of time will lead to low magnesium levels,[14] magnesium is what is needed to encourage the correct utilization of calcium by the body to increase bone strength.[15] Researchers estimate currently that the ratio should be two parts calcium to one part magnesium.[16]
Without magnesium, calcium is not fully utilized, and under absorption problems may occur leading to arthritis, osteoporosis, menstrual cramps, and some premenstrual symptoms.
In contrast to skeletal muscle, cardiac muscle cannot contract in the absence of extracellular calcium ions as well as extracellular potassium ions. In this sense, it is intermediate between smooth muscle, which has a poorly developed sarcoplasmic reticulum and derives its calcium across the sarcolemma; and skeletal muscle which is activated by calcium stored in the sarcoplasmic reticulum (SR). The reason for the calcium dependence is due to the mechanism of calcium-induced calcium release (CICR) from the SR that must occur under normal excitation-contraction (EC) coupling to cause contraction.
According to Dr. Sarah Mayhill, “Calcium and magnesium compete for absorption and so too much calcium in the diet will block magnesium absorption. Our physiological requirement for calcium to magnesium is about 2:1. In dairy products the ratio is 10:1. So, consuming a lot of dairy products will induce a magnesium deficiency.”
A diet high in dairy and low in whole grains can lead to excess calcium in the tissues and a magnesium deficiency.[17]– Dr. Nan Kathryn Fuchs
pH
The general theory behind increased calcium intake is that calcium will combat excess acidity, thus helping to promote good health. This is only half true: While the body uses calcium as a buffer, excess calcium can also promote soft-tissue calcification. Too much calcium running amuck through your body is the real danger of excess acidity. It is far better to increase your intake of other buffers such as magnesium, which will safely buffer excess acidity without causing calcifications. Of course, eating a so-called alkaline diet and limiting your intake of acidic minerals such as phosphorus may also help. Acidic minerals can contribute to calcifications. In essence, the real danger of excess acidity is the leeching of calcium that it causes. Simply put, excess acidity equals soft-tissue calcifications.
The chemical reaction of magnesium is alkaline (acid binding). It regulates the acid-alkaline balance of the body.– Dr. H. Ray Evers
According to Dr P Kaye, Emergency Department, Bristol Royal Infirmary, UK, “Magnesium acts as a smooth muscle relaxant by altering extracellular calcium influx and intracellular phosphorylation reactions. It may also attenuate the neutrophilic burst associated with inflammatory bronchoconstriction by attenuating mast cell degranulation. The principal trigger for this degranulation is a rise in intracellular calcium, which is antagonised by magnesium. It has been shown experimentally to augment the bronchodilatory effect of salbutamol and to inhibit histamine induced bronchospasm. Magnesium should be used as a safe, easy to administer and effective second line agent in acute severe asthma.[18]
Medical authorities claim that the widespread incidence of osteoporosis and tooth decay in western countries can be prevented with a high calcium intake. However the opposite is true. Asian and African populations with a very low intake of about 300 mg of calcium daily have very little osteoporosis. Bantu women with an intake of 200 to 300 mg of calcium daily have the lowest incidence of osteoporosis in the world. In western countries with a high intake of dairy products the average calcium intake is about 1000 mg. With a low magnesium intake, calcium goes out of the bones to increase tissue levels, while a high magnesium intake causes calcium to go out of the tissues into the bones. Thus high magnesium levels leads to bone mineralization.
Some gynecologists believe that one of the first organs to calcify is the ovaries, leading to pre-menstrual tension.
Dr. Karen Kubena, associate professor of nutrition at Texas A & M University indicates that even if you monitor your magnesium level like a maniac, you’re still at risk for migraines if your calcium level is out of whack. It seems that higher than normal blood levels of calcium cause the body to excrete the excess calcium, which in turn triggers a loss of magnesium. “Let’s say you have just enough magnesium and too much calcium in your blood. If calcium is excreted, the magnesium goes with it. All of a sudden, you could be low in magnesium,” says Dr. Kubena.
As a general rule, acid substances tighten; and alkaline substances relax. Magnesium is alkaline and relaxes the body from tightness, tension, stiffness, spasms, twitches, tics or jerkiness as in nervousness, anxiety, anger, fear, agitation, headaches, muscle cramps, menstrual cramps, arthritis, insomnia, constipation, heart palpitations, irregular heartbeats, high blood pressure, eye twitches, acne, plaque on teeth, plaque on heart and arteries due to cholesterol build-up, plaque on the brain [Alzheimer’s]. Magnesium acts as a natural gate or valve in the brain synapses that regulates influx of calcium into postsynaptic calcium channels from presynaptic neurons in parts of the brain that are involved in mood and behavior such as the hippocampus. With inadequate magnesium (calcium toxicity), this function becomes altered and irritability, anxiety, depression, ADHD, mania, hypo-mania, bi-polar disorder, hyper-excitability and hyper-emotionality, and perhaps some psychoses, result.
A pH less than 5.3 indicates an inability to assimilate vitamins or minerals. Due to the alkalinity of minerals, they loosen tumors, including fibroid tumors, endometriosis, cysts, moles, warts, skin tags, and other growths, and cause them to release their toxins. Magnesium should be used to buffer acid pH, not the calcium that is being leached from the bones.
Magnesium taken in proper dosages can solve the problem of calcium deficiency.– Dr. Nan Kathryn Fuchs
Experts say excessive calcium intake may be unwise in light of recent studies showing that high amounts of the mineral may increase risk of prostate cancer. “There is reasonable evidence to suggest that calcium may play an important role in the development of prostate cancer,” says Dr. Carmen Rodriguez, senior epidemiologist in the epidemiology and surveillance research department of the American Cancer Society (ACS). Rodriguez says that a 1998 Harvard School of Public Health study of 47,781 men found those consuming between 1,500 and 1,999 mg of calcium per day had about double the risk of being diagnosed with metastatic (cancer that has spread to other parts of the body) prostate cancer as those getting 500 mg per day or less. And those taking in 2,000 mg or more had over four times the risk of developing metastatic prostate cancer as those taking in less than 500 mg.
The recommended daily allowance (RDA) of calcium is 1,000 mg per day for men and 1,500 mg for women.
Later in 1998, Harvard researchers published a study of dairy product intake among 526 men diagnosed with prostate cancer and 536 similar men not diagnosed with the disease. That study found a 50% increase in prostate cancer risk and a near doubling of risk of metastatic prostate cancer among men consuming high amounts of dairy products, likely due, say the researchers, to the high total amount of calcium in such a diet. The most recent Harvard study on the topic, published in October 2001, looked at dairy product intake among 20,885 men and found men consuming the most dairy products had about 32% higher risk of developing prostate cancer than those consuming the least. Dr. Panagiota N. Mitrou, of the National Cancer Institute, Rockville, Maryland, and colleagues found the same thing, that increased consumption of calcium and dairy products raises the risk of prostate cancer.
Treatment with Sodium Thiosulfate
Sodium thiosulfate (STS) is a calcium chelating agent with antioxidant properties.– Dr. Carlos E. Araya

Figure 1. (A) Initial three-phase bone scan demonstrating soft tissue accumulation
in thighs, distal femur, proximal tibia, and forearms. There is intense uptake in the
myocardium and early accumulation in the lungs. (B) Three months later, the
calcium deposition in the thighs and forearms is less significant. However,
there still is calcification in the heart, lungs, and para-articular surfaces.
Sodium thiosulphate results in the formation of calcium thiosulphate in the urine, a compound with much higher solubility than the other calcium salts (phosphate, oxalate). Thus, sodium thiosulphate could not only inhibit further nephrocalcinosis, but in some degree it could contribute to decalcification of renal parenchyma[19].
The beneficial effects of sodium thiosulfate (STS) are thought to be due in part to its ability to enhance the solubility of calcium deposits. STS has a small molecular weight of 248 (Na2S2O3) and in patients with normal renal function has a serum half-life of 15 min. STS facilitates the mobilization of calcium from vessels affected by calcium deposits.
Intravenous STS seems beneficial, has mild adverse effects, and is well tolerated in children and young adults. STS dosage was 25 g/1.73 m2 per dose intravenously.– Dr. Carlos E. Araya
Dr. Carlos E. Araya et al[20] successfully used this relatively nontoxic substance, which been reported as adjuvant treatment of several conditions involving disorders of calcium homeostasis. Yatzidis described its benefits by decreasing the rate of new kidney stone development in 34 patients with recurrent calcium urolithiasis. Prompted by these excellent results, intravenous STS was administered after hemodialysis to three patients with ESRD and tumoral calcinosis for a period of 6 to 12 mo. Two of the patients had regression of the calcified mass as well as improved motility of the affected joints. STS was given for a period of 9 yr to a patient with nephrocalcinosis as a result of renal tubular acidosis type 1. There was no further deterioration of his condition, and the discontinuation of the medication was accompanied by recurrence of renal colic. See later chapter for the full story on sodium thiosulfate.
Body pH and Calcium
Many health care professionals believe there is only one disease. And that one disease is acidosis. The wastes produced from food are highly acidic and acidosis is one of the main contributors that lead to the aging process and various illnesses. Acid waste is excreted from the human body in the form of urine or sweat. But the wastes not excreted will be circulating around in the blood, in the body. This acidic waste will gradually accumulate somewhere in our capillaries blood vessels, and eventually clog them up. Also as a consequence of this, the cells of the human body will be deprived of their supply of oxygen and essential nutrients, rendering these cells inactive in reproduction. That’s the main reason why people age. Moreover, with the capillary blood vessels clogged up, the function of every organ in the human body accumulating acidic waste will begin to deteriorate, causing serious illnesses in the long run.
One of the first warning signs of an acidic biological terrain is calcium deposits. Our dietary intake of calcium is not keeping up with the calcium buffering needed and we are actively pulling calcium from our bones and teeth. It all works like a little train, from the bones to the fluids and cells, to the blood. As our biological terrain becomes acidic, our pH level drops. When this happens we start losing calcium out of the blood, the bones, and the tissues. This is a safety mechanism. Now your biological terrain’s oxygen level drops leaving you tired and fatigued, allowing fungus, mold, parasites, bad bacteria, and viral infections to flourish and gain a hold throughout the body. It is interesting to note that you often won’t have just some of these invaders. If you have Candida you will likely have bad bacteria, fungus, and parasites because they all flourish in the same terrain.
Mild acidosis can cause such problems as:
- Cardiovascular damage, including the constriction of blood vessels and the reduction of oxygen.
- Weight gain, obesity and diabetes.
- Bladder and kidney conditions, including kidney stones.
- Immune deficiency.
- Acceleration of free radical damage, possibly contributing to cancerous mutations.
- Premature aging.
- Osteoporosis; weak, brittle bones, hip fractures and bone spurs.
- Joint pain, aching muscles and lactic acid buildup.
- Low energy and chronic fatigue.
A recent seven year study conducted at the University of California, San Francisco, on 9,000 women showed that those who have chronic acidosis are at greater risk for bone loss than those who have normal pH levels. The scientists who carried out this experiment believe that many of the hip fractures prevalent among middle aged women are connected to high acidity caused by a diet rich in animal foods and low in vegetables. This is because the body borrows calcium from the bones in order to balance pH.
American Journal of Clinical Nutrition
The biggest problem scientists have found is that over time the human body becomes depleted of calcium. A compound called mono-ortho-calcium phosphate is the chemical buffer for the blood. This buffer maintains the alkaline level (or the lack of acidity) in your blood. Without it you would die. If the acidity level of your blood changes even slightly, you die immediately! But in order to supply enough calcium for buffering we must have enough calcium being absorbed from our diet. If not, our body will simply rob the needed calcium from our bones and teeth.
The more acidic we become, the harder it is for oxygen to be present, so our biological terrain also becomes more anaerobic. Without adequate oxygenation, unfriendly bacteria, viruses, molds, and fungus can live and prosper. Then our cells cannot carry on their life-giving functions in a very efficient manner because our biological chemical reactions need oxygen.
[1] New York State Department of Health; http://www.health.state.ny.us/diseases/conditions/osteoporosis/qanda.htm
[2] Accu-Cell Nutrition; Calcium and Magnesium http://www.acu-cell.com/acn.html
[3] Nephrol Dial Transplant (2002) 17: 336-339 Cardiovascular calcification in end-stage renal disease. Isidro B. Salusky1, and William G. Goodman2 1 Department of Pediatrics and 2 Department of Medicine, UCLA School of Medicine, Los Angeles, CA, USA
[4] J Am Coll Cardiol, 2002; 39:695-701. J Am Coll Cardiol, 2002; 39:695-701 American College of Cardiology Foundation.
[5] Signs and symptoms of hypercalcemia may include:
| • Nausea | • Fatigue |
| • Vomiting | • Lethargy |
| • Stomach Pain | • Moodiness |
| • Constipation | • Irritability |
| • Anorexia | • Confusion |
| • Excessive thirst | • Extreme muscle weakness |
| • Dry mouth or throat | • Irregular heart beat |
| • Frequent Urination | • Coma |
[6] Magnes Res. 2008 Jun;21(2):77-82. A connection between magnesium deficiency and aging: new insights from cellular studies. Killilea DW, Maier JA. Nutrition and Metabolism Center, Children’s Hospital Oakland Research Institute.
[7] Nutrition Action Healthletter, June, 1993
[8] American Journal of Clinical Nutrition, 1995; 61 (4)
[9] Osteoporosis International 1993;3(3)
[10] Braun, J, Oldendorf, M, Moshage, W, et al. Electron beam computed tomography in the evaluation of cardiac calcification in chronic dialysis patients. Am J Kidney Dis 1996; 27:394.
[11] Goodman, WG, Goldin, J, Kuizon, BD, et al. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med 2000; 342:1478.
[12]Arch Intern Med. 2005;165:327-332
[13] Eades M, Eades A, The Protein Power Lifeplan, Warner Books, New York, 1999
[14] Camara-Martos, F. and M.A. Amaro-Lopez, Influence of Dietary Factors on Calcium Bioavailability. Biological Trace Element Research, 2002. 89: p. 43-52
[15] Jones, G., M. Riley, and T. Dwyer, Maternal Diet during pregnancy is associated with bone mineral density in children: a longitudinal study. European Journal of Clinical Nutrition, 2000. 54: p. 749-756
[16] Celotti, F. and A. Bignamini, Dietary Calcium and Mineral/Vitamin Supplementation: a controversial problem. The Journal of International Research, 1999(27): p. 1-14
[17] The source of menstrual cramps may come from eating too much cheese, yogurt, ice cream or milk, combined with insufficient whole grains and beans. Or it could come from taking too much calcium without enough magnesium. Modifying diet and increasing magnesium supplementation may allow menstrual cramps to disappear.
[18] Kaye, P. O’Sullivan, I.The role of magnesium in the emergency department. Emergency Department, Bristol Royal Infirmary, Bristol, UK Emerg Med J 2002; 19:288-291
[19] Yatzidis H. Successful sodium thiosulphate treatment for recurrent calcium urolithiasis. Clin Nephrol1985; 23: 63–67
[20] Sodium Thiosulfate Treatment for Calcific Uremic Arteriolopathy in Children and Young Adults. Carlos E. Araya, Robert S. Fennell, Richard E. Neiberger, and Vikas R. Dharnidharka. Division of Pediatric Nephrology, Department of Pediatrics, University of Florida College of Medicine, Gainesville, Florida. Clin J Am Soc Nephrol 1: 1161-1166, 2006. http://cjasn.asnjournals.org/cgi/content/full/1/6/1161
Dr.Sircus is a reader-supported publication. To receive new posts and support my work, consider becoming a free or paid subscriber.
Subscribe now



comments