Magnesium serves hundreds of important functions in the body and one of them has to do with the efficiency of red blood cells and their capacity to carry oxygen. Researchers have investigated the effect of dietary magnesium (Mg) deficiency on the nutritive utilization and tissue distribution of iron (Fe). Magnesium deficient diet leads to significant decreases in the concentration of red blood cells (RBC), hemoglobin and eventually a decrease in whole blood Fe. In fact we find many ways in which magnesium deficiency leads to problems with oxygen transport and utilization.[i]
Chronic Mg deficiency has also been shown to increase copper absorption and concentrations in plasma, muscle, kidney, and liver.[ii] Magnesium is involved with the transport of ions, amino acids, nucleosides, sugars, water and gases across the red blood cell membrane. Magnesium levels drop more slowly in red blood cells than in the serum.[iii]
A study by ARS physiologist Henry C. Lukaski and nutritionist Forrest H. Nielsen reveals important findings on the effects of depleted body magnesium levels on energy metabolism. Lukaski is assistant director of ARS’s Grand Forks Human Nutrition Research Center. The data shows that magnesium deficient people used more oxygen during physical activity -their heart rates increased by about 10 beats per minute. “When the volunteers were low in magnesium, they needed more energy and more oxygen to do low-level activities than when they were in adequate-magnesium status,” says Lukaski.[iv]
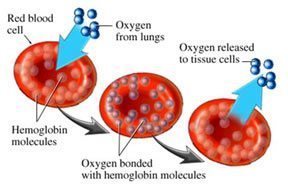
“The transport of oxygen in blood is undertaken by hemoglobin, the largest component of red blood cells. This protein collects oxygen in respiratory organs, mainly in the lungs, and releases it in tissues in order to generate the energy necessary for cell survival. Hemoglobin is one of the most refined proteins because its evolution and small mutations in its structure can produce anemia and other severe pathologies,” publishes the Institute for Research in Biomedicine (IRB Barcelona).
They continue, “More than a hundred years of study have led to the knowledge that hemoglobin uses mechanisms of cooperatively to optimize its function; that is to say, to collect the greatest amount of oxygen possible in the lungs and release it in tissues. These mechanisms of cooperatively are related to changes in the structure of the hemoglobin protein.”
The structure of hemoglobin is easily compromised by heavy metals like mercury (as are all sulfur bearing proteins[v] like insulin etc). In my book NewParadigms in Diabetic Medicine we nail down the mercury sulfur bond death destruction scenario. You can bet your last medical dollar on the fact that high magnesium and selenium status is protective of red blood cells and thus of total oxygen carrying capacity.
The mechanism whereby red cells maintain their biconcave shape has been a subject of numerous studies. One of the critical factors for the maintenance of biconcave shape is the level of red cell adenosine triphosphate (ATP) levels. The interaction of calcium, magnesium and ATP with membrane structural proteins exerts a significant role in the control of shape of human red blood cells.[vi] Magnesium enhances the binding of oxygen to haem proteins.[vii] The concentration of Mg2+ in red cells is relatively high but free Mg2+ is much lower in oxygenated red blood cells then in deoxygenated ones. This suggests some kind of magnesium pump where oxygen climbs aboard the red cells and magnesium jumps off only to have to jump right back on the red cells again.
Dr. L.O. Simpson asserts that Fatigue Immune Deficiency Syndrome (CFIDS), results from “insufficient oxygen availability due to impaired capillary blood flow.” This would naturally reflect to the mitochondria who would be having their O2 deprivation problems. In healthy people, most red blood cells are smooth-surfaced and concave-shaped with a donut-like appearance. These discocytes have extra membranes in the concave area that give them the flexibility needed to move through capillary beds, delivering oxygen, nutrients, and chemical messengers to tissue and removing metabolic waste, such as carbon dioxide and lactic acid.
Red blood cells are also known as erythrocytes. They have a unique shape known as a biconcave disk. A biconcave disk is like a donut where the hole doesn’t go all the way through. The biconcave disk shape increases the surface area of the cell which allows for a greater area for gas exchange.
Abnormal magnesium deprived red blood cells lack flexibility that allow them to enter tiny capillaries. These nondiscocytes are characterized by a variety of irregularities, including surface bumps or ridges, a cup or basin shape, and altered margins instead of the round shape found in discocytes. When people become ill or physically stressed (more magnesium deficient), a higher percentage of discocytes transform into the less flexible nondiscocytes.
Magnesium stimulates the movement of oxygen atoms from the bloodstream to the cells.
Magnesium and zinc prevent the binding of carbon monoxide/CO to haem which otherwise binds 25,000 times more strongly than does oxygen. The dissociation of oxygen is also helped by magnesium, because it provides an oxygen adsorption isotherm which is hyperbolic. It also ensures that the oxygen dissociation curves are sigmoidal which maximizes oxygen saturation with the gaseous pressure of oxygen (Murray et al pp. 65-67).
Oxygen dissociation with increased delivery to the tissues is increased by magnesium through elevation of 2,3-bisphosphoglycerate/DPG (Darley, 1979) Magnesium stabilizes the ability of the phorphyrin ring to fluoresce. Free-radical attack of haemoglobin yields ferryl haemoglobin [HbFe4+] (D’Agnillo and Alayash, 2001), which is inhibited by magnesium (Rock et al, 1995).
Magnesium prevents blood vessels from constricting, thus warding off rises in blood pressure, strokes and heart attacks. Magnesium inhibits the release of thromboxane, a substance that makes blood platelets stickier..[5]– Dr. Jerry L. Nadler
Low red blood cell magnesium levels, a more accurate measure of magnesium status than routine blood analysis, have been found in many patients with chronic fatigue. Red blood cell (RBC) deformability is an important factor in determining movement of red blood cells through the microcirculation. Intravenous magnesium therapy over a 24-hour period has been shown to increase RBC-deformability even in pregnancies with normal RBC-deformability. An increase of RBC-deformability with magnesium administration offers therapeutic benefit for the treatment of reduced blood flow seen in most cases of preeclampsia.[viii].
D F Treacher and R M Leach also teach, “Oxygen transport from environmental air to the mitochondria of individual cells occurs as a series of steps. The system must be energy efficient (avoiding unnecessary cardiorespiratory work), allowing efficient oxygen transport across the extravascular tissue matrix. At the tissue level, cells must extract oxygen from the extracellular environment and use it efficiently in cellular metabolic processes.” No matter what kind of medicine one practices this is good basic medicine to understand and appreciate.
Patients with chronic fatigue syndrome (CFS) have low red blood cell magnesium. The physiological concept of fatigue as a consequence of inadequate oxygen delivery is widely accepted tying oxygen carrying capacity directly to magnesium.
Magnesium-deficiency studies on the kidneys have shown intraluminal calcareous deposits in the corticomedullary area and damage to the tubular epithelium. Damage to the kidneys from magnesium deficiency creates a situation that intensifies the magnesium deficit. Micropuncture studies have shown that most active renal tubular reabsorption of magnesium occurs at sites that are potentially damaged by magnesium deficiencies meaning these conditions can cause renal tubular magnesium wasting. Both hyper-parathyroidism and hypervitaminosis D increase blood and thus urinary loads of calcium and thus cause even further magnesium loss.
Most renal reabsorption of magnesium occurs in the proximal tubule and the thick ascending limb of the loop of Henle. In hypomagnesemic patients, the kidney may excrete as little as 1 mEq/L of magnesium. Additionally, magnesium may be removed from bone stores in times of deficiency.
Primary renal disorders cause hypomagnesemia by decreased tubular reabsorption of magnesium by the damaged kidneys. This condition occurs in the diuretic phase of acute tubular necrosis, postobstructive diuresis, and renal tubular acidosis.
Drugs may cause magnesium wasting.
Diuretics (eg, thiazide, loop diuretics) decrease the renal threshold for magnesium reabsorption in addition to wasting of potassium and calcium.
Cisplatin causes dose-dependent kidney damage in 100% of patients receiving this drug.
Pentamidine and some antibiotics also cause renal magnesium wasting.
Fluoride poisoning similarly causes hypomagnesemia.
[i] Influence of magnesium deficiency on the bioavailability and tissue distribution of iron in the rat. The Journal of Nutritional Biochemistry, Volume 11, Issue 2, Pages 103-108
[ii] J. Agric. Food Chem., 1997, 45 (10), pp 4023–4027 DOI: 10.1021/jf970011k – http://pubs.acs.org/doi/abs/10.1021/jf970011k
[iii] http://www.jbc.org/cgi/reprint/122/3/693.pdf
[iv] http://www.agclassroom.org/teen/ars_pdf/family/2004/05lack_energy.pdf
[v] It has long been known that the sulfur contents of hemoglobins of different species vary. Therefore one or both of the sulfur containing amino acids must exist in different quantities in the various globins.
[vi] http://bloodjournal.hematologylibrary.org/cgi/reprint/44/4/583.pdf
[vii] Terwilliger and Brown, 1993; Takenhiko and Weber; Wood and Dalgleish, 1973
[viii] http://www.informahealthcare.com/doi/abs/10.1081/PRG-45767?cookieSet=1&journalCode=hip
Dr.Sircus is a reader-supported publication. To receive new posts and support my work, consider becoming a free or paid subscriber.
Subscribe now




comments