
A glass of water at pH 9 can have low, medium or high ALKALINITY – and everything in between, depending on what’s dissolved in it. It is what is in one’s water that makes all the difference. Bottom line—you can have high pH water (highly alkaline) and have just about no alkalinity to speak of – meaning the water hardly buffers the body’s acid condition at all. It’s not high pH alkaline water we need, it’s water with high levels of ALKALINITY.
There was something I found in our drinking water that made me sit up and take notice about this crucial subject. I tested our water and to my dismay, it was slightly acid but putting only a pinch of sodium bicarbonate into a half gallon moved it to an alkaline pH. It seems that nearly everyone, public and professional alike, is confused over the terms “alkaline” and “alkalinity.”
To put it simply, it is not enough to drink high pH alkaline water – in fact pH is not a big factor at all when one is trying to alkalize the body (just “think” lemon juice, apple cider vinegar and fresh green juices – all of which have an acid pH but are still great for alkalizing!). The key to addressing excessive metabolic acids and building alkalizing reserves in our body fluids and tissues is the amount of alkalinity (alkaline mineral compounds) that is consumed.
Water expert Robert Slovak wrote, “Baking soda is still beloved! It delivers the goods – real alkalinity – while most popular alkaline pH drops from well-known “health experts” are a hoax at $35-$40 for pennies worth of caustic chemical in 2oz of water. Just note that for some people baking soda (sodium bicarbonate) may supply too much sodium so they can substitute potassium bicarbonate or mix the two.”
For those concerned about acidity in distilled or RO water Slovak tells us that it’s more misinformation, “The truth is, when atmospheric CO2 reaches equilibrium in distilled or RO water (forming weak carbonic acid) the pH goes slightly lower (say pH 6.5) but there is virtually no acid-buffering capacity! As soon as the distilled or RO water hits your saliva or stomach-acid, its pH is rapidly readjusted and it takes on a different pH and buffering capacity. To say that distilled water and RO water are highly acidic is simply wrong – a marketing gimmick to favor alkaline water! The weak carbonic acid that forms simply cannot acidify the body. Furthermore, just a little baking soda dust on the end of your finger can completely neutralize a glass of distilled or RO water.”
“The pH of water, by itself, has no "teeth" unless it has buffering capacity. I can easily give you drinking water with a pH less than 7 that can neutralize more acid than water with a pH of 10! That’s how un-intuitive this subject is and why people are confused. High pH ionized water, without accompanying alkalinity (bicarbonates, carbonates and hydroxides), does little but waste money.” continued Slovak.
Even when the pH of water is very high, the lack of sufficient alkalinity (alkalizing minerals) makes it impossible to neutralize acid in the stomach to initiate the production of bicarbonate in the bloodstream (the Alkaline Tide effect). An alkaline ionizer barely adds any alkalinity to the water (but raises the pH) so the only real acid neutralizing capacity is from the alkalinity already in your tap water! It is a waste of money to alkalize the body using alkaline ionized water – not when baking soda is so available and inexpensive.
Alkalinity is the water’s capacity to resist changes in pH that would make the water more acidic. This capacity is commonly known as “buffering capacity.” For example, if you add the same weak acid solution to two vials of water – both with a pH of 7, but one with no buffering power (e.g. zero alkalinity) and the other with buffering power (e.g. an alkalinity of 450 mg/l) – the pH of the zero alkalinity water will immediately drop while the pH of the buffered water will barely change at all.
The alkalinity of natural water is determined by the composition of soil and bedrock through which it passes. The main sources for natural alkalinity are rocks, which contain carbonate, bicarbonate, and hydroxide compounds. Borates, silicates, and phosphates also may contribute to alkalinity. Limestone is rich in carbonates, so waters flowing through limestone regions or bedrock-containing carbonates generally have high alkalinity – hence good buffering capacity. Conversely, surface water (e.g., streams, ponds, lakes) and underground zones rich in granites and some conglomerates and sandstones may have low alkalinity and therefore poor buffering capacity.
People using alkaline ionizers in low mineral areas (many city supplies using surface water or river sources) are especially misled because the water from their alkaline ionizers can still be set to a high pH. But, there is too little alkalinity present to make a health difference. They would be much better off with 1/2 tsp. of baking soda added to the water. All users of water ionizers should consider adding sodium bicarbonate to their water if they are looking for stronger alkalizing effects.
"Cancerous tissues are acidic, whereas healthy tissues are alkaline, Water splits into H+ and OH- ions, if there is an excess of H+, it is acidic; if there is an excess of OH- ions, then it is alkaline."
Dr. Otto Heinrich Warburg
1931 Nobel Prize Winner for discovering – The Real Cause of Cancer
pH is related to the hydrogen ions in water and stands for “potential of hydrogen.” Alkalinity is a measure of the capacity of water to neutralize acids. It measures the presence of carbon dioxide, bicarbonate, carbonate, and hydroxide ions that are naturally present in water. At normal drinking water pH levels, bicarbonate, and carbonate are the main contributors to alkalinity. As we can see in the graph below. the higher the CO2 the more alkaline the water at a given pH.
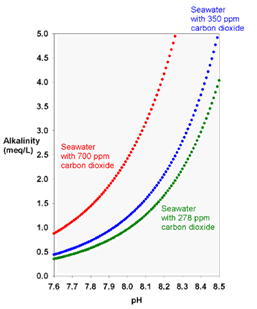
In the chemistry of natural waters, there are several types of alkalinity that are encountered. Each of these is a measure of how much acid (H+) is required to lower the pH to a specific level. The reason that aquarists measure alkalinity is that in normal seawater, most alkalinity consists of bicarbonate and carbonate. Consequently, alkalinity is an indication of whether or not adequate bicarbonate is present in the water. Sodium bicarbonate is the main alkaline buffer in our blood. Alkaline buffers supplied from outside the body, like drinking bicarbonate water, results in a net gain of alkalinity in our body.
The main chemical species that contribute to alkalinity in seawater are bicarbonate and carbonate. The table below (from “Chemical Oceanography” by Frank Millero; 1996) shows the contribution to alkalinity from the major contributors in seawater at pH 8.
| Chemical Species |
Relative Contribution To Alkalinity |
|
HCO3– (bicarbonate) |
89.8 |
|
CO3– (carbonate) |
6.7 |
|
B(OH)4–(borate) |
2.9 |
|
SiO(OH)3–(silicate) |
0.2 |
|
MgOH+ (magnesium monohydroxylate) |
0.1 |
|
OH–(hydroxide) |
0.1 |
|
HPO4–and PO4—(phosphate) |
0.1 |
Carbon dioxide has a specific solubility in water as carbonic acid (H2CO3). At any given pH there is an exact mathematical relationship between H2CO3 and both bicarbonate and carbonate. For example, at a pH of about 9.3 in freshwater (about 8.4 in seawater) the carbonate concentration is 100 times that of the carbonic acid. At higher pH this multiplier rises, and there is consequently more bicarbonate and carbonate present.
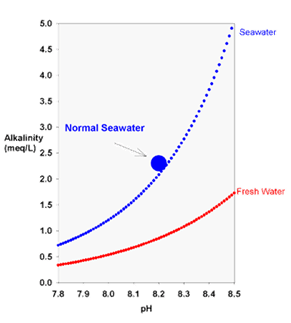
The theoretical relationship between carbonate alkalinity and pH for seawater (blue) and freshwater (red) equilibrated with the atmosphere (350 ppm carbon dioxide).
Bicarbonate water is the healthiest water to drink. It is critical to see that alkalinity does not depend strictly on pH. pH measures the degree of alkalinity but not its quantity. It is like the relationship between temperature and heat.
The ultimate water treatment system is water treated with magnesium bicarbonate. One can take distilled water and Reverse Osmosis water and bring that water to perfection by adding magnesium bicarbonate (which also restructures the water) or in a less costly formula, sodium bicarbonate with sprays of a high grade magnesium oil added to the water. Turning water into medicine is what my book Water Based Medicine is all about.
Dr.Sircus is a reader-supported publication. To receive new posts and support my work, consider becoming a free or paid subscriber.
Subscribe now




comments